Rigicon,Inc. announces that the U.S. Food and Drug Administration (FDA) has cleared Rigi10™ Malleable Penile Prosthesis for implantation into the corpora cavernosa of the penis for men who are diagnosed with erectile dysfunction. The prosthesis is implanted to provide adequate penile rigidity for sexual intercourse.
Rigi10™ is easy to implant and easy to use. The Flexible Rod Technology™ enables higher bending angles with no spring-backs. Rigi10™ is offered in 5 different diameters and 2 sizes for a better fit to the patient’s anatomy.
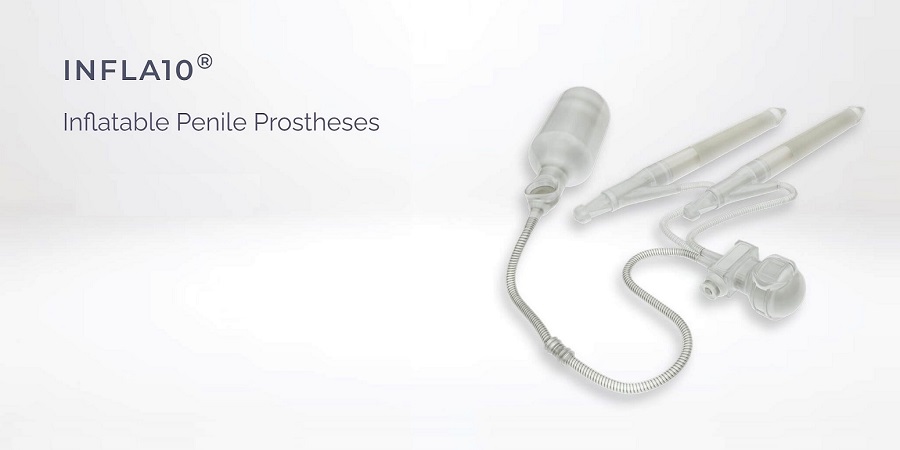
Infla10® is the next generation inflatable penile prosthesis designed with years of experience in urology. Infla10® incorporates a hydrophilic coating on all external component surfaces to facilitate rapid and strong absorption of the solution on the device and promote easier device implantation. Innovative Rapid-Pump™ mechanism offers greater ease and speed for patients when inflating and deflating the prosthesis. Infla10®AX version offers the patients anatomical girth and length expansion addressing concerns regarding post-operative penile shortening.
Penile Prosthesis is for treating erectile dysfunction, a condition affecting more than 200 million men annually worldwide, with nearly 50% of men over 60 suffering from moderate to severe symptoms.[1,2]
About Erectile Dysfunction
Erectile Dysfunction, or ED, is the inability to achieve or sustain an erection suitable for sexual intercourse. ED is actually a common male sexual disorder and may maintain a permanent course as some men cannot have any erection for the remaining part of their life. ED might have a deep impact on the quality of life and social relationships of the patient.
ED may be basically caused by various diseases and disorders including heart disease, diabetes, pelvic area damage due to surgery, accidents or radiation therapy, benign prostatic hyperplasia (BPH, which is non-cancerous enlargement of the prostate tissue), disorders associated with low testosterone levels, Parkinson’s disease, and other neurologic diseases.
About Malleable Penile Prosthesis (Semi-Rigid Penile Prosthesis)
Malleable penile prosthesis is a medical device that allows an impotent male to achieve an erection. The malleable implant consists of two rods that are always hard but pliable. All components are concealed within the body and cannot be seen from the outside. The penile implant cylinders reside in the penis on either side. No tissue is removed to place the rods; the rods simply occupy spaces that were previously filled with blood, when one was potent. The rods do not disrupt the flow of urine or ejaculate. The rods do not alter the sensation of the penis.
About Inflatable Penile Prosthesis (Three – Piece Inflatable Penile Prosthesis)
Inflatable devices, the most common type of penile implant used, can be inflated to create an erection and deflated at other times. Three-piece inflatable implants use a fluid-filled reservoir implanted under the abdominal wall, a pump and a release valve placed inside the scrotum, and two inflatable cylinders inside the penis. (Source:Mayo Clinic)
Penile implant surgery is considered a gold standard therapy for men with erectile dysfunction.
About Rigicon®
Rigicon® designs and manufactures innovative urological implants with 30 years experience on Urology. We focus on creating a comprehensive product portfolio for urologist(s) around the world. Our commitment is to deliver high quality and innovative urology products to our valued patients and physicians.
[1]US Census Bureau.
[2] Aytac IA et al (1996) – The likely worldwide increase in erectile dysfunction between 1995 and 2025and some possible policy consequences. BJUI, 84: 50-6.